Clinical trial program
VitroScan’s clinical trial program brings our ex vivo micro‑tumor platform directly into the clinic. The trials integrate patient‑derived micro‑tumor assays alongside traditional clinical endpoints. Eligible participants undergo minimally invasive tissue collection, enabling the generation of individualized micro‑tumors that are screened against investigational therapeutics, standard‑of‑care therapies, and rational combination regimens.
Key Objectives
- Predictive validation – Correlation of ex vivo responses with in‑patient outcomes across diverse tumor types, thereby establishing the assay as a predictive tool for precision oncology.
- Risk mitigation – Early identification of non‑responders to minimize exposure to ineffective therapies and to optimize resources for both sponsors and patients.
- Accelerated decision making – Provision of actionable biomarker and efficacy data that inform go/no‑go milestones, dose selection, and cohort expansion strategies, ultimately shortening development timelines.
- Regulatory alignment – Generation of reproducible, high-quality datasets that satisfy FDA and EMA regulatory requirements for IND/CTA submissions, companion‑diagnostic development and label‑expansion submissions.
Collaborative partnerships
VitroScan works closely with academic medical centers and pharmaceutical sponsors to co‑design protocols that leverage predictive insights in clinical trials. These partnerships foster a shared ecosystem wherein scientific innovation, patient benefit, and treatment success align.
Impact on patient care
By aiming to embed predictive testing into the clinical trial workflow, VitroScan will empower oncologists and patients to personalize treatment decisions, enhance response rates, and improve quality of life for patients. The program also builds a growing evidence base that supports the broader adoption of ex vivo testing as a standard component of precision oncology.
Proven clinical translations
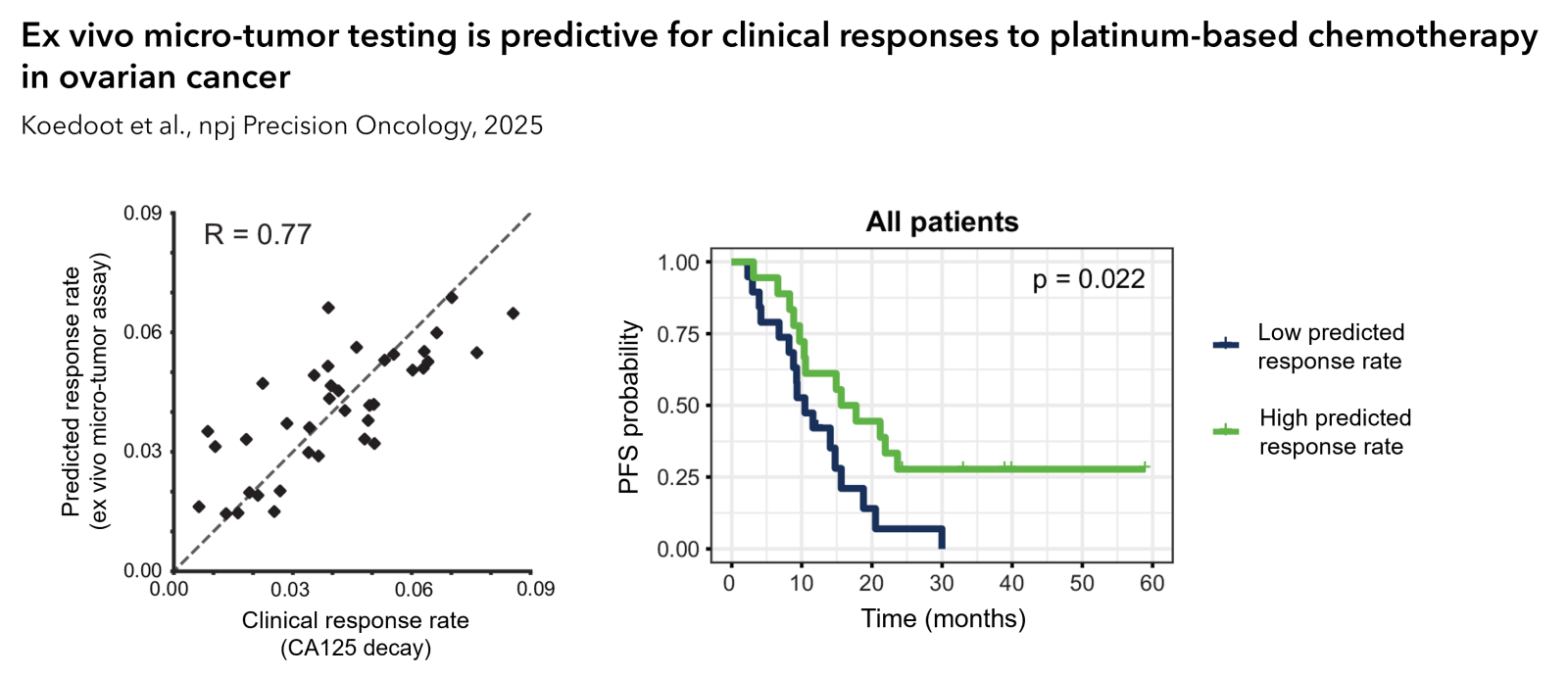
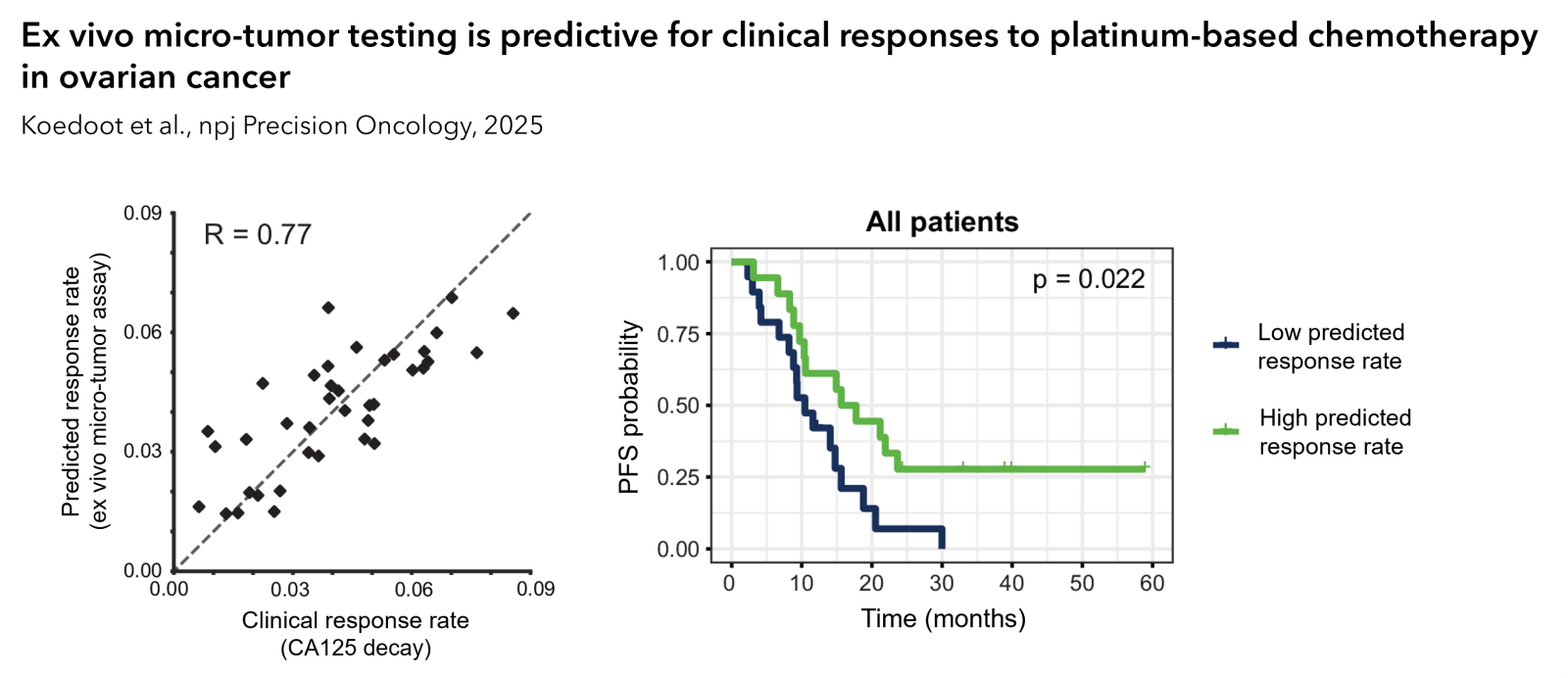
The ex vivo platform is proven predictive for clinical responses to platinum-based chemotherapy (Koedoot et al., npj Precision Oncology, 2025). In ovarian, lung and bladder cancer, clinical correlations have been established for chemo- and targeted therapies. Moreover, immunotherapy responses observed ex vivo correlate with clinical biomarkers such as PD-L1 expression.
Explore all downloadable publications including info sheets, scientific posters and scientific publications for more insights
Connect with VitroScan
Contact us for more information on how our testing solutions can help you reduce risk and maximize success in your drug development process or for any other inquiries!